Do Alkaline Earth Metals Occur Freely in Nature
Because of their reactivity the alkaline metals are. The alkaline earth elements are metallic elements found in the second group of the periodic table.

Alkaline Earth Metal Properties List Reactivity Britannica
Alkaline earth metals do not occur freely in nature due to their high level of reactivity.
. These metals have only one electron in their outer shell. This means that they are only found in compounds in the. All alkaline earth elements have an oxidation number of 2 making them very reactive.
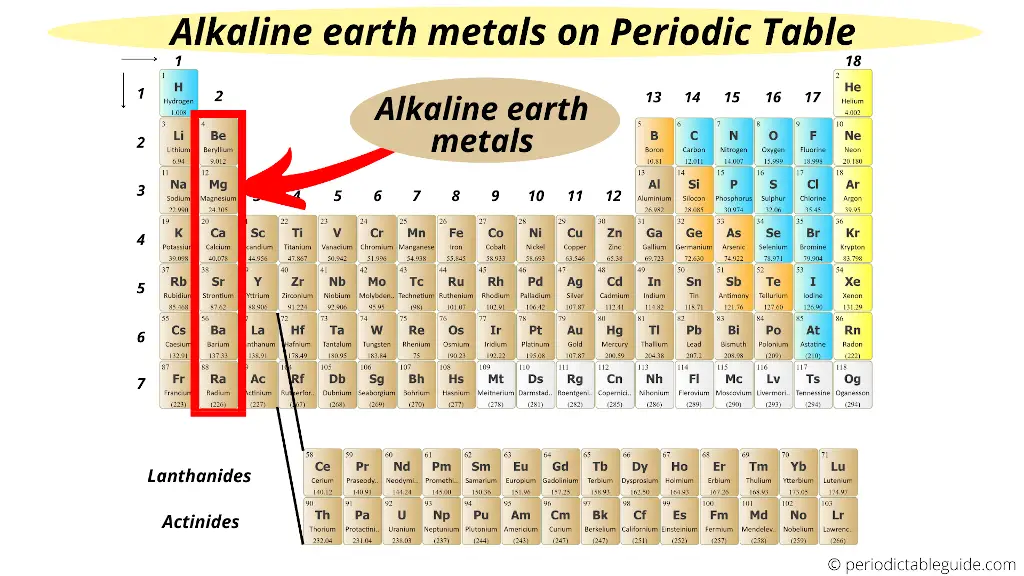
All of the alkaline earth metals except magnesium and strontium have at least one naturally occurring radioisotope. Beryllium Be Magnesium Mg Calcium Ca Strontium Sr Barium Ba and Radium Ra. Group II elements include.
All the alkaline earth metals readily lose their two outermost electrons to form cations with a 2 charge. The alkaline earth elements are metallic elements found in the second group of the periodic table. The alkaline earth metals are the elements that correspond to group 2 of the modern periodic table.
Being very reactive alkaline earth metals also do not occur in free state. Beryllium Be Magnesium Mg Calcium Ca Strontium Sr Barium Ba and Radium Ra. All alkaline earth elements have an oxidation number of 2 making them very reactive.
How do you store alkali metals. Alkaline Earth Metals Transition Metals Other Metals Metalloids Non-Metals Halogens Noble Gases Rare Earth Elements The alkali metals found in group 1 of the periodic table formerly known as group IA are very reactive metals that do not occur freely in nature. This group of elements includes beryllium magnesium calcium strontium barium and radium.
They do not occur freely in nature and react readily with halogens. Transition Elements These metals have high densities strength and are resistant to corrosion. The outer portion of the earth was originally in the form of silicates and alumino-silicates of alkaline earth metals.
To minimize contact with oxygen and water alkali metals must be stored in an airtight container. Do alkaline earth metals occur freely in nature. Magnesium and calcium are ubiquitous and essential to all known living organisms.
Group II elements include. The alkali metals are consist of different chemical elements such as lithium which has a symbol Li sodium Na potassium K caesium Cs rubidium Rb and francium Fr. The alkaline earth elements are metallic elements found in the second group of the periodic table.
Because of their reactivity the alkaline metals are not found free in nature. Because of their reactivity the alkaline metals are not found free in nature. Very reactive Do not occur freely in nature 3 11 19 37 55 87 1 valence electron.
For example all alkaline earth metals are silvery-white. The compounds of these metals occur widely in nature. Salt formers 7 valence electrons Exist in all 3.
Similar to alkaline metals these elements also do not occur freely in nature and they are also very reactive. See full answer below. Alkaline earth metals are found in the second group of the periodic table.
It is about Alkali metals. Based on the attached document the question is referring to Question number 12. The elements of this group are quite similar in their physical and chemical properties.
What are Alkaline Earth Metals. Elements classified as Alkali Metals are very reactive metals that do not occur freely in nature. Occurrence of Alkaline earth Metals.
Magnesium and calcium are very abundant in earths crust. Alkaline earth metals are in the second group of the periodic table. Similar to alkaline metals these elements also do not occur freely in nature and they are also very reactive.
Very reactive 2 valence electrons Do not occur freely in nature 4 12 20 38 56 88.

Where Are Alkaline Earth Metals Found On The Periodic Table


No comments for "Do Alkaline Earth Metals Occur Freely in Nature"
Post a Comment